The Global Oncology Initiative at WashU has been awarded a $2.7 million grant from the U.S. National Cancer Institute to develop and test a novel AI-powered imaging device to monitor treatment response in patients with Kaposi sarcoma (KS) in East Africa. Led by Assistant Professor Dr. Thomas Odeny in the Division of Oncology, the five-year study aims to improve care and treatment outcomes for KS, a leading cause of cancer-related death among men in East Africa. Despite declines in overall incidence of KS in the U.S., incidence among Blacks/African Americans has remained stable or increased. Worse, Black/African American people with KS have significantly higher mortality than other groups.
In Africa, where the global burden of KS is highest, the traditional manual measurement method using a ruler to measure skin lesions is both time-consuming and impractical for routine clinical care, particularly in busy low- and middle-income clinics.
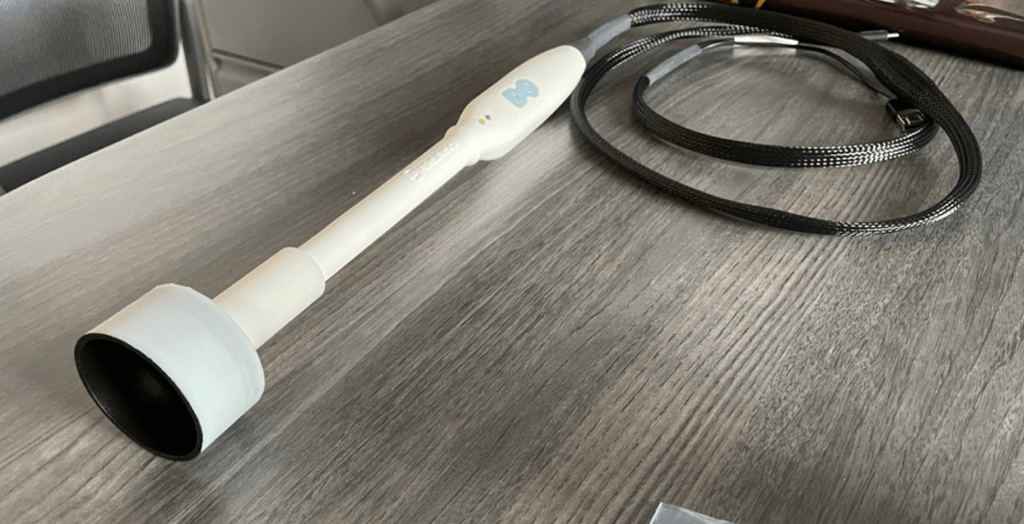
“Current methods for monitoring KS treatment response are outdated and imprecise, with little change over the past three decades,” says Dr. Odeny, a specialist in treating HIV-associated cancers. “AI-powered precision imaging, such as our SkinScan 3D, has the potential to transform the manual approach, providing more accurate and reliable results for KS treatment monitoring.”
Developed by Pensievision, the new SkinScan 3D device offers a more accurate and efficient solution. Utilizing liquid lens technology and artificial intelligence, the device captures high-resolution 3D images of KS lesions, incorporating their height and volume, which enables more precise tracking of treatment response.